Age-related macular degeneration is a leading cause of vision loss for people over 60. SYFOVRE, the first treatment for geographic atrophy, is now approved with another drug under FDA review and others in development, offering hope for the future.
As someone living with age-related macular degeneration (AMD), you understand the impact this condition can have on your vision. You may have already gone through the different stages of dry AMD, from early to intermediate, and now may be facing or soon will be facing geographic atrophy.
Hope is here. A new treatment option for geographic atrophy SYFOVRE (pegcetacoplan injection) is now approved by the FDA. And another will have the FDA review completed by August this year.
Further, other drugs targeting different mechanisms, such as antioxidative stress, visual cycle modulation, gene therapy, and cell-based therapy, are also under investigation and offer hope for the future.
Stay informed and stay hopeful. The future of treatment for geographic atrophy in macular degeneration looks bright.
What is geographic atrophy in AMD?
Dry age-related macular degeneration (AMD) is a common eye disorder in people over 50 years old, and it is the leading cause of vision loss in people over 60. More than 85% of individuals living with AMD have the dry form, which is caused by the gradual death of retina cells in the macula, a crucial part of the retina responsible for central vision.
The condition progresses through three stages: early, intermediate, and late. In the early stage, there is usually no noticeable vision loss, and it may be detected during a comprehensive eye exam. However, in the intermediate stage, changes or loss of central vision may occur. In the late stage, vision is usually severely impacted.
Late AMD can be further divided into two types: wet and dry, with the latter also known as geographic atrophy (GA).
Did you know that Geographic atrophy has many synonyms, such as late/advanced dry AMD, atrophic AMD, end-stage dry AMD, late-stage non-neovascular AMD, or late nonexudative AMD?
Geographic atrophy is the most severe form of dry AMD, leading to vision loss in an estimated 1.5 million people in the USA and up to 5 million people worldwide. It is characterized by a progressive enlargement of lesions and retina cell death in the macula. Unfortunately, its progression is irreversible. [2]
How does complement system overactivity lead to geographic atrophy?
Dry age-related macular degeneration is a condition in which small white or yellowish deposits, known as drusen, develop on the retina, beneath the macula. This leads to a gradual deterioration or degeneration of the macula over time.
In the early stages of the condition, small drusens may be identified in the back of the eye. However, as the disease progresses to the intermediate stage, the drusens become larger and more numerous, and changes in the retina pigment become noticeable.
Overactivity of the complement pathway, a part of the immune system responsible for identifying and destroying foreign substances or intruders, is thought to cause the formation of drusens. And several studies indicate that complement activation plays an important role in the development and progression of geographic atrophy.
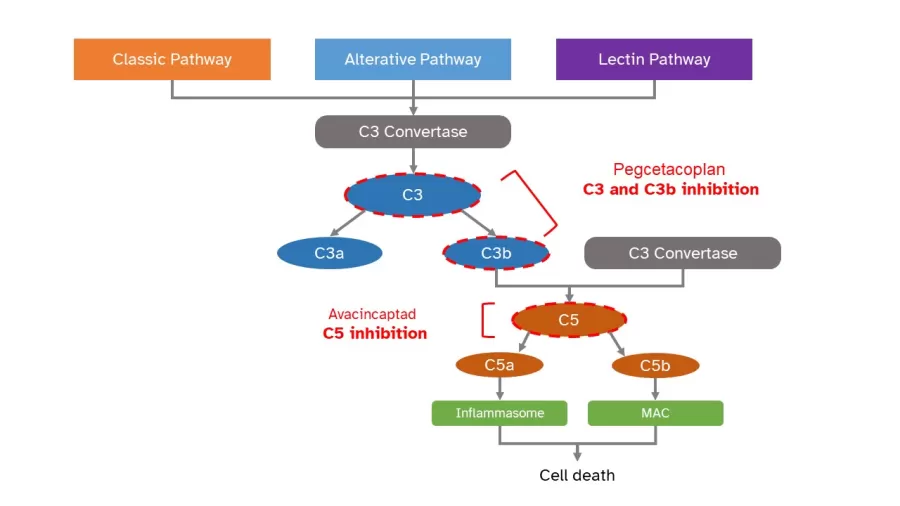
A complement cascade is part of the immune system that helps protect the body against harmful intruders, such as germs and viruses. It's made up of a group of proteins in the blood that works together to fight off infections. The cascade can be turned on in three ways: classical, alternative, and lectin pathways.
When the complement cascade is activated, it creates different pieces that can damage and kill germs, viruses, or even cells. One of these pieces, called C3, is split into two smaller pieces, C3a and C3b. As the cascade continues, another piece called C5 is split into C5a and C5b. These pieces created from the cascade can combine to create structures – inflammasome and membrane attack complex (MAC) – that can damage retina cells, leading to cell death and vision loss in dry AMD. [2]
Is there any treatment for dry AMD?
On Feb 17, 2023, Apellis Pharmaceuticals announced that the FDA had approved SYFOVRE (pegcetacoplan injection) for treating geographic atrophy (GA) in people with age-related macular degeneration.
SYFOVRE is the first drug approved by the FDA to treat this condition, and it works by stopping the action of C3 and C3b.
The treatment is administered as an eye injection every 25 to 60 days, and studies showed that it could reduce the growth of GA lesions better than a sham injection (a type of placebo that is like a real injection), and it demonstrated increasing treatment effects over time.
In its Phase III trials, SYFOVRE reduced the rate of GA lesion growth by 18 to 22% (monthly injection) or 17 to 18% (every two months) compared to sham through 24 months. And the most significant reduction happened between months 18-24, with up to a 36% reduction in lesion growth in the monthly treatment group. [1]
The most common side effects are discomfort, neovascular age-related macular degeneration, vitreous floaters, and conjunctival hemorrhage.
The company noted that SYFOVRE would be available in the US starting in March; the European Medicines Agency is currently reviewing an application for marketing authorization, with a decision expected in early 2024; and an application has also been submitted to Health Canada. [1]
What are the upcoming treatments for geographic atrophy in macular degeneration?
There are many drugs under development for the treatment of geographic atrophy. Avacincaptad pegol, is another treatment that was submitted to the FDA for review. It also works on the complement system but prevents the formation of C5a and C5b.
In its Phase III trials, avacincaptad pegol reduced the geographic atrophy lesion area by about 27% compared to sham injection. A 6.7% choroidal neovascularization in the avacincaptad group and 4.1% in the sham group were reported. The expected FDA review completion date is August 19th, 2023. [5]
Other drugs being investigated for the treatment of geographic atrophy include elamipretide, risuteganib, ALK-001, GT005, and OpRegen. These drugs target different mechanisms, such as antioxidative stress, visual cycle modulation, gene therapy, and cell-based therapy. [4]
And they provide opportunities to further improve outcomes for people living with late AMD and help preserve their vision.
Key takeaways
With SYFOVRE FDA-approved for geographic atrophy, healthcare providers now have the first effective tool for this vision-threatening condition.
Furthermore, there are also other drugs under FDA review or in development that target a different part of the complement system or mechanisms, such as C5, antioxidative stress, visual cycle modulation, gene therapy, and cell-based therapy.
Stay positive, stay informed, and keep your eyes on the prize - a brighter future awaits!
Want to stay connected on the newest happening? Join me
Pinterest board: https://www.pinterest.com/clearsightcorner/
- FDA Approves SYFOVRE™ (pegcetacoplan injection) as the First and Only Treatment for Geographic Atrophy (GA), a Leading Cause of Blindness (Source: https://investors.apellis.com/news-releases/news-release-details/fda-approves-syfovretm-pegcetacoplan-injection-first-and-only)
- Desai, Dhaval, and Pravin U Dugel. “Complement cascade inhibition in geographic atrophy: a review.” Eye (London, England) vol. 36,2 (2022): 294-302. doi:10.1038/s41433-021-01765-x
- Efficacy of intravitreal pegcetacoplan in patients with geographic atrophy (GA): 12-month results from the phase 3 OAKS and DERBY studies.(Source: https://iovs.arvojournals.org/article.aspx?articleid=2779604)
- Geographic Atrophy Treatments In The Pipeline for 2022 (Source: https://www.ophthalmologyadvisor.com/slideshow/retina-vitreous/potential-therapies-for-geographic-atrophy-in-amd/)
- Iveric Bio Announces FDA Accepts New Drug Application and Grants Priority Review for Avacincaptad Pegol for the Treatment of Geographic Atrophy (Source: https://www.businesswire.com/news/home/20230216005773/en/Iveric-Bio-Announces-FDA-Accepts-New-Drug-Application-and-Grants-Priority-Review-for-Avacincaptad-Pegol-for-the-Treatment-of-Geographic-Atrophy)

Comments